West Bengal PSC Previous Year Chemistry Questions with Answers (Part-1)
In this post we list West Bengal Public Service Commission's previous year Chemistry related questions with detailed solution. We arrange these questions topic wise. This is Part-1.
Actually this is the part of WBPSC Previous Year Science Series. Under this we solve the Science Question subject wise.
WBPSC Previous Year Physics Question with Answer
Part-1 , Part-2 , Part-3 , Part-4
WBPSC Previous Year Computer Questions with Answers
Environment
Q. Which of the following has no greenhouse effect? [WBPSC Miscellaneous Exam- 2012(1st)]
- CH4
- N2O
- SO2
- H2O
C. SO2
Explanation: Here, we assume H2O as water vapour.
CH4(Methane), N2O(Nitrous Oxide), water vapour is greenhouse gas.
SO2(Sulphur dioxide) is not a direct greenhouse gas. It is considered as indirect greenhouse gas because it shows greenhouse effect when coupled with carbon element.
Greenhouse gasses are the gases which trap the heat energy in the atmosphere.
Q. Acid rains occur due to [WBPSC Miscellaneous Exam- 2012(1st)]
- Presence of CO2 in the atmosphere
- Presence of CO and CO2 in the atmosphere
- Presence of Ozone in the atmosphere
- Presence of SO2 and SO3 in the atmosphere
D. Presence of SO2 and SO3 in the atmosphere
Explanation:
When rain or any kind of natural precipitation (rain, snow, fog etc.) includes a large number of acidic component, that is broadly known as acid rain.
SO2 , SO3 , NO, NO2 are mainly responsible for acid rain. In fact, these gasses ultimately form H2SO4(Sulphuric Acid), HNO3 (Nitric Acid).
Effects of Acid rain
- Acid rain has harmful impact on soils, forests.
- It can kill small insects, fresh-water fish etc.
- Acid rain is the cause of stone cancer.
- Taj Mahal (made of white marble) is turning yellow due to this acid rain.
Q. Bio gas consist of
[WBCS Preliminary Exam- 2019]
- Carbon monoxide, Methane and Hydrogen
- Carbon dioxide, Methane and Hydrogen
- Carbon monoxide, Ethane and Hydrogen
- Carbon dioxide, Ethane and Hydrogen
B. Carbon dioxide, Methane and Hydrogen
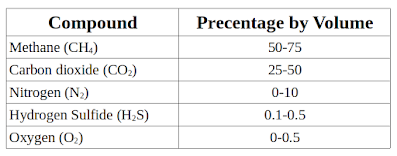
Explanation: Bio gas is a mixture of gasses produced by the breakdown of organic matter by anaerobic bacteria.
Typical composition of bio gas
Atomic Structure
Q. An atom of an element differs from an atom of one of its isotopes in the number of
[WBPSC Miscellaneous Exam- 2012(1st)]
- Neutrons in nucleus
- Proton in nucleus
- Valence electron
- Proton outside the nucleus
A. Neutrons in nucleus
Explanation: If two atoms of an element have same number of proton but different number of neutron, two atoms are called as isotopes of each other.
Atomic, Molecular Theory and Equivalent weight
Q. How many times is chlorine gas heavier than air? [WBPSC Miscellaneous Exam- 2012 (2nd)]
- 1.27
- 2.50
- 3.81
- 5.0
B. 2.50
Explanation: The density of chlorine gas is approximately 2.5 times greater than that of air.
Q. How many grams of NaOH are needed to make 100ml of a 0.5M solution of NaOH? (Atomic weight: Na=23; O=16; H=1) [WBCS Preliminary Exam- 2015]
- 2
- 20
- 4
- 1
A.2
Explanation: Molarity is written as "M". Molarity is a type of expressing the concentration.
1 Molar solution means 1 mole solute is dissolve in 1 litre solution.
Mass of 1 mole of NaOH is (23+16+1)g=40g.
Here concentration is 0.5M.
So, 0.5 mole NaOH is dissolved in 1litre=1000ml solution.
0.5mole=(40×0.5)g=20g
Therefore, for 1000ml solution 20g needed.
For 100ml solution((20/1000)×100)g=2g needed.
Q. Which of the following fertilizers has more nitrogen content? [WBCS Preliminary Exam-2019]
- Urea
- Ammonium Nitrate
- Potassium Nitrate
- 5.Ammonium Phosphate
A. Urea
Explanation:
Atomic weight: C=12.01; O=16; N=14.008; H=1.008; K=39.10; P=30.975
Urea= CO(NH2)2
Molecular weight
={12.01+16+(14.008+2×1.008)×2)
=60.058
Ammonium Nitrate= NH4NO3
Molecular weight
=(14.008+4×1.008+14.008+3×16)
=80.048
Potassium Nitrate= KNO3
Molecular weight
=(39.10+14.008+3×16)
=101.108
Ammonium Phosphate= (NH4)PO4
Molecular weight
=(14.008+4×1.008)×3+30.975+4×16
=149.095
Chemical Energetics (Thermodynamics etc.)
Q. A reaction is spontaneous when [WBCS Preliminary Exam-2017]
- ΔG=−Ve
- ΔH=−Ve
- ΔS=+Ve
- ΔS=−Ve
A.ΔG=−Ve
Explanation: Gibbs free energy is expressed as
ΔG=ΔH−TΔS
ΔG=Change in Gibbs free energy
ΔH= Change in enthalpy
T= Temperature in Kelvin scale
ΔS= Change in entropy
ΔG=+Ve ⇒the reaction is non-spontaneous
ΔG=0 ⇒reaction is in equilibrium
ΔG=−Ve⇒ the reaction is spontaneous
Periodic Table
Q. Which of the following pairs is from the same group of Periodic table?
[WBPSC Miscellaneous Exam- 2012 (1st)]
- Mg, Ca
- Mg, K
- Mg, Cu
- Mg, I
A. Mg, Ca
Explanation: In Periodic table horizontal rows are known as Period and vertical columns are called as Group.
Atomic number of Mg is 12 and Ca is 20.
Q. The strongest electropositive element is
[WBCS Preliminary Exam-2017]
- Cs
- Li
- Mg
- K
A. Cs
Explanation:
Electropositivity: It is a tendency of an atom to donate electrons for forming a positively charged ion.
- Caesium(Cs) is the most electropositive stable element.
- Fluorine(F) is the least electropositive element.
Molecular Structure and Chemical Bonding
Q. Which of the following pairs is isoelectronic?
[WBPSC Miscellaneous Exam- 2012 (2nd)]
- N2, CO
- NO, CO
- CO, CN−
- O2, CN−
A. N2, CO; C. CO, CN−
Explanation: Isoelectronic atoms or molecules have same number of electron or have same electronic structure.
Carbon(C) has 6 electrons.
Nitrogen(N) has 7 electron.
Oxygen(O) has 8 electron.
Anion having 1 negative charge has 1 more electron.
N2, CO
N2 has (7+7)=14 electrons.
CO has (6+8)=14 electrons.
NO, CO
NO has (7+5)=15 electrons.
CO has (6+8)=14 electrons.
CO, CN−
CO has (6+8)=14 electrons.
CN− has (6+7+1)=14 electrons.
O2, CN−
O2 has (8+8)=16 electrons.
CN− has (6+7+1)=14 electrons.
So, N2 and CO are isoelectronic.
And CO and CN− are isoelectronic.
Q. Geometry of SF4 is
[WBCS Preliminary Exam-2017]
- square planar
- tetrahedral
- octahedral
- see-saw
D. see-saw
Explanation:
Atomic number of S is 16.
Atomic number of F is 9.
No. of bond pair=4
NO. of lone pair=1
Hybridization: sp3d
Geometrical shape: see-saw
Properties of Gas
Q. Ratio of the rms speed and the most probable speed for molecules in an ideal gas is
[WBCS Preliminary Exam-2021]
- √3:1
- 1:√3
- √3:√2
- √2:√3
C.√3:√2
Explanation:
For an Ideal Gas:
=C:Cm=√3:√2.
Alloy
Q. Brass is a mixture of
[WBCS Preliminary Exam-2019]
- Copper & Zinc
- Copper & Tin
- Copper, Nickle & Zinc
- Copper, Aluminium & Mg
A. Copper & Zinc
Explanation:
Constituent material of alloy:
Brass:
Copper(Cu): 60-80%
Zinc(Zn): 40-20%
Bronze:
Copper(Cu): 75-90%
Tin(Sn): 25-10%
German silver:
Copper(Cu): 50%
Nickle(Ni): 20%
Zinc(Zn): 30%
Duralumin:
Aluminium(Al): 97%
Copper(Cu): 4%
Magnesium(Mg): 0.5%
Manganese(Mn): 0.5%
Q. Solder used for joining metal parts together is an alloy of
[WBCS Preliminary Exam-2021]
- Fe and Cu
- Fe and Zn
- Sn and Cu
- Sn and Pb
D. Sn and Pb
Explanation:
Solder is an alloy of Tin(Sn) and Lead(Pb).
Electrolysis, Ores and Minerals
Q. Which of the following is an or of aluminium?
[WBPSC Miscellaneous Exam- 2012 (2nd)]
- hematite
- dolomite
- bauxite
- carnalite
C. bauxite
Explanation:
- Bauxite (Al2O3,2H2O) is an ore of Aluminium.
- Hematite (Fe2O3) is an ore of iron(Fe).
- Dolomite (CaMg(CO3)2) is an ore of Magnesium(Mg).
- Carnalite (KMgCl3.6(H2O)) is an ore of Potassium(K) and Magnesium(Mg).
Q. The metal first discovered by man is
[WBPSC Miscellaneous Exam- 2012 (2nd)]
- iron
- copper
- aluminium
- gold
B. copper
Explanation: The metal, copper was first discovered by man.
Q. Which one among the following is the main ingredient in cement?
[WBCS Preliminary Exam-2019]
- Gypsum
- Limestone
- Clay
- Ash
B. Limestone
Explanation: The main ingredient in cement is Calcium Oxide(Lime). Presence of lime in cement is 60-65%.
In-fact lime is obtained from limestone.
Chemical name of limestone is Calcium carbonate (CaCO3).
The second most important ingredient in cement is Silica(SiO2). In cement presence of Silica is 17-25%.
Gypsum, clay are also used in cement making. But these are used in small quantity.
Oxidation and Reduction
Q. Bleaching action of Chlorine is by
[WBCS Preliminary Exam-2018]
- decomposition
- hydrolysis
- reduction
- oxidation
D. Oxidation
Explanation: In bleaching action of Chlorine, water plays crucial role. In bleaching process, Chlorine first reacts with water and produce nascent Oxygen.
Nascent Oxygen is highly reactive. It combines with colour materials. Hence, colour material become colourless.
WBPSC Previous Year CHEMISTRY Question with Answers Part-2
WBPSC Previous Year SCIENCE Science
WBPSC Previous Year Physics Questions with Answers Part-1
WBPSC Previous Year Physics Questions with Answer Part-2
WBPSC Previous Year Computer Questions with Answers
If your have any queries, you may comment bellow.
Thank You.
Wish You, You Meet Your Goal










Comments
Post a Comment