West Bengal PSC Previous Year CHEMISTRY Questions with Answers (Part-2)
In this post we list West Bengal Public Service Commission's previous year CHEMISTRY related questions with detailed solution. Here we arrange previous questions topic wise. This post is Part-2. Visit Part-1.
Actually this is the part of our WBPSC Previous Year Science Series.
Under this science series we list and solve WBPSC Previous Year PHYSICS, CHEMISTRY, BIOLOGY, MATHEMATICS and COMPUTER questions topic wise.
Properties of Matter
(Acid, Base, Metal, Non-metal, Gas)
Q. Which of the following acids can dissolve gold? [Miscellaneous Exam- 2012(1st)]
- HNO3
- H2SO4
- H2SeO4
- HClO4
C. H2SeO4
Explanation: Hot concentrated H2SeO4 (Selenic acid) reacts with gold and can dissolve it.
Generally, Aquaregia is used for dissolving gold, platinum.
Aquaregia is a formed by mixing Nitric acid (HNO3) and Hydrochloric acid (HCl) in 1:3 ratio.
Q. "Vanishing colour" may be prepared by mixing phenolphthalein with [WBPSC Miscellaneous Exam- 2012(1st)]
- Carbon dioxide, water
- Sulphurous acid
- dilute solution of caustic soda
- dilute ammonia solution
D. dilute ammonia solution
Explanation: Phenolphthalein is a chemical compound which is used as an indicator in acid-base titration.
Phenolphthalein turns colourless in acidic solution or neutral solution and pink in basic solution.
Dilute ammonia solution is basic in nature. Hence, ammonia solution with phenolphthalein turns pink. As water evaporates, ammonia(gas) also evaporates, eventually, phenolphthalein turns colourless.
In this question, among 4 given options , only ammonia solution is basic solution which evaporates in normal temperature. Caustic soda (NaOH) is basic in nature but solid in home temperature.
Q. Water gas is a mixture of [WBPSC Miscellaneous Exam- 2012(1st)]
- CO, CH4, C2H2
- CO, CO2 and methane
- CO and H2
- CO and N2
C. CO and H2
Explanation: Water gas is mixture of Carbon monoxide(CO) and Hydrogen(H2)
Q. Soaps are [WBPSC Miscellaneous Exam- 2012(2nd)]
- sodium and potassium salts of higher fatty acids
- sodium salts of sulphuric acids
- high molecular mass alcohols
- mixture of glycerol and phenol
A. sodium and potassium salts of higher fatty acids.
Explanation: soaps are sodium and potassium salts of higher fatty acids.
Q. When chlorine reacts with excess ammonia the products are [WBPSC Miscellaneous Exam- 2012(2nd)]
- NH4Cl, NCl3
- NH4Cl, N2
- NCl3, HCl
- N2, HCl
B. NH4Cl, N2
Explanation:
Ammonia is oxidized by chlorine.
In the reaction of chlorine with excess Ammonia, Ammonia is oxidized and form Nitrogen. On the other hand Chlorine is reduced and form Hydrochloric acid.
So, if we write a single equation for this, it would be
Q. The compound that can not be kept in glass vessel because it reacts with glass is [WBCS Preliminary Exam- 2015]
- HNO3
- HCl
- HF
- HBr
C. HF
Explanation: HF (Hydrofluoric acid) cannot be stored in glass because it corrode the glass.
Actually, a compound named silicates react with HF and get dissolved.
Q. Which among the following is called "Laughing Gas" popularly? [WBCS Preliminary Exam- 2016]
- Nitric oxide
- Nitrous oxide
- Nitrogen penta oxide
- Nitrogen
B. Nitrous oxide
Explanation: Nitrous oxide (N2O) is popularly known as "Laughing Gas".
Nitrous oxide is significantly used in medical treatment especially in surgery and dentistry for its anesthetic and pain reducing effect.
Q. Which of the following is used in beauty parlours for hair stetting?
[WBCS Preliminary Exam-2019]
- Chlorine
- Sulphur
- Phosphorus
- Silicon
B. Sulphur
Explanation: Sulphur is used in beauty parlour for hair setting.
Q. Select the one having pH<7
[WBCS Preliminary Exam-2019]
- Lemon juice
- Lime water
- Human blood
- Antacid
A. Lemon juice
Explanation:
pH scale is used to measure the acidity and basicity of a solution. Generally, the range of scale is 0-14.
pH=7 represent the solution is neutral.
pH<7 means, the solution is acidic in nature.
pH>7 indicates the solution is a base.
We know that lemon juice is acidic in nature. So, pH of lemon juice is less than 7.
Lime water and Antacid are basic in nature.
Human blood is slightly basic and pH of human blood is about 7.35 to 7.45.
Q. To protect tooth decay we are advised to brush our teeth regularly. The nature of the tooth paste used is
[WBCS Preliminary Exam-2019]
- acidic
- neutral
- basic
- corrosive
C. basic
Explanation:
Acidic environment in the mouth corrodes the teeth enamel.
For maintaining healthy teeth we should keep the mouth environment near to neutral.
Many things we eat actually turn mouth into acidic.
Tooth paste is usually basic in nature which in fact, neutralize the excess acid and keep teeth healthy.
Q. In terms of thermal conductivity (k) and electrical conductivity (δ), diamond has
[WBCS Preliminary Exam-2021]
- low k and high δ
- high k and low δ
- high k and high δ
- low k and low δ
B. high k and low δ
Explanation:
Diamond has high thermal conductivity (k) and low electrical conductivity (δ).
Q. Dry ice means
[WBCS Preliminary Exam-2021]
- Ice at -23℃
- Ice at 4℃
- Solid SO2
- Solid CO2
D.Solid CO2
Explanation: Solid CO2 (Carbon dioxide) is known as Dry ice.Dry ice sublimates (turn into vapour directly from solid) at -78.5℃.
Dry ice is commonly used in preserving food (like meat etc.).
Q. The chopping of an onion makes one cry because of the chemical containing
[WBCS Preliminary Exam-2021]
- Sulphur
- Chlorine
- Bromine
- Nitrogen
A. Sulphur
Explanation: Onion absorbs Sulphur from the soil and they use it to make Sulphur-rich amino acid.
When onion are cut, we actually cut many cells of onion. Eventually many enzymes are released.
Enzymes react with sulphur-rich amino acid and ultimately form Syn-Propanethial-S-Oxide. this chemical is volatile, it floats up from the chopping board to our eyes and makes us cry.
Organic Chemistry
Q. Saturated hydrocarbons are termed Paraffin because [WBPSC Miscellaneous Exam- 2012(1st)]
- They are much less reactive
- They are highly reactive
- They are insoluble in water
- They are non-combustible
A. They are much less reactive.
Explanation: Paraffin is a soft colourless solid derived petroleum, coal.
The name "Paraffin" is derived from Latin. "Paraffin" literally means "lacking affinity" or "lacking reactivity".
In saturated hydrocarbons, all C-C bonds are single bond i.e. sigma covalent bond. These type of bonds are very stable and tough to break. Tat is why, saturated hydrocarbons are much less reactive.
Hence, in Chemistry, often, saturated hydrocarbons are termed as Paraffin.
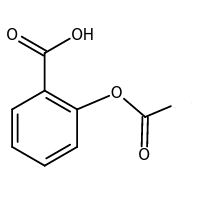
Q. Chemically Aspirin is : [WBCS Preliminary Exam-2015]
- Acetyl salicylic acid
- Sodium salicylate
- Methyl salicylate
- Ethyl salicylate
A. Acetyl salicylic acid
Explanation: Chemically Aspirin is Acetyl salicylic acid.
Aspirin is generally used to reduce pain, fever or inflammation.
Q. The term PVC used in plastic industry stand for [WBCS Preliminary Exam-2016, 2018]
- Phospho vinyl chloride
- Poly Vinyl Carbonate
- Poly Vinyl Chloride
- Phospho Vanadium Chloride
C. Poly Vinyl Chloride
Explanation: The term PVC used in plastic industry stands for Poly Vinyl Chloride.
PVC is a polymer. Monomer of PVC is Vinyl chloride.
PVC is used in making of pipe, insulation of electric wire etc.
Q. Conversion of CH3C≡CH to CH3CH=CH2 needs [WBCS Preliminary Exam-2017]
- Lindlar catalyst
- H2/ Pd
- NaBH4
- LiAlH4
A. Lindlar catalyst
Explanation: Lindlar catalyst is used to convert alkynes to alkenes (without further reduction into alkanes).
Here, CH3C≡CH is alkyne.
and CH3CH=CH2 is alkene.
Hence, conversion of CH3C≡CH to CH3CH=CH2 needs LIndlar catalyst.
Q. Conversion of RBr to RMgBr requires [WBCS Preliminary-2017]
- Mg/ dry ether/ N2-atmosphere
- Mg/ moist ether/ N2-atmosphere
- Mg/ ethanol/ N2-atmosphere
- Mg/ dry ether/ O2-atmosphere
A. Mg/ dry ether/ N2-atmosphere
Explanation:
RMgx is known as Grignard reagent.
R= alkyl or aryl group.
X= halogen
Preparation of Grignard reagent
Reaction environment should be entirely dry and there should be no water vapour, O2, CO2.
So, conversion of RBr to RMgBr requires Mg/ dry ether/ N2-atmosphere.
Q. The faster SN1 reaction is of the followings: [WBCS Preliminary Exam-2017]
- MeO-CH2-Cl
- Me-CH2-Cl
- Ph-CH2-CH2-Cl
A. MeO-CH2-Cl
Explanation: MeO-CH2-Cl will react faster in SN1 reaction.
Q. Chemical name of Vinegar is [WBCS Preliminary Exam-2018]
- Sodium nitrate
- Dilute acetic acid
- Chloride of lime
- Calcium
B. Dilute acetic acid
Explanation: Vinegar is an aqueous solution of acetic acid (CH3COOH).
Thank You
Wish, You Meet Your Goal
WBPSC Previous Year CHEMISTRY Questions with Answers Part-1
WBPSC Previous Year SCIENCE Question with solution


Comments
Post a Comment